A 20 Minute Introduction to Cluster Randomised Trials
Posted on 23rd August 2013 by Tim Hicks

Who is it for?
Students of medicine or from the clinical sciences and professions allied to medicine with an interest in different study designs.
What will I learn and how?
The key features of a cluster Randomised Trial with a stepwise progressive approach and a ‘concept check’ question as each new element is introduced.
How long will it take?
Approximately 20 minutes.
Contents
Introduction
What is a Cluster Randomised Trial?
When is it appropriate to use a cluster randomised trial?
Considerations for trial design and analysis
Bringing it all together – Real world example
Summary
References
Answers
Introduction
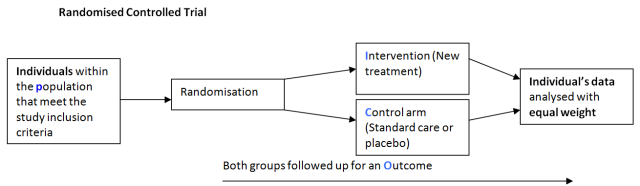
There are many different study designs used by researchers to investigate a research question each with it own particular merits and pitfalls. Typically when considering randomised controlled trials we think of a study where individuals who satisfy the inclusion criteria volunteer to participate in a study.
Individuals are then randomised to either an intervention (new treatment) or control (Standard care or placebo) and followed up over time for the outcome of interest. The results are then analysed as two distinct groups where equal weight is given to the result of each individual (statistical independence).
What is a Cluster Randomised Trial?
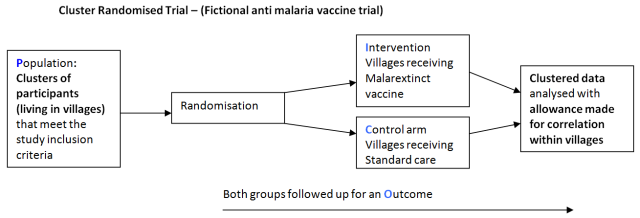
A cluster randomised trial is a study design which randomises groups of participants to each arm of a study rather than individuals. This is done when it would be difficult give a new treatment to an individual within a community or social group without it affecting the outcome in the standard care arm of the study.
When is it appropriate to use a cluster randomised trial?
For example if individuals from the same village were recruited to trial a new vaccine in a study using individual randomisation, vaccinated individuals would not be susceptible to the disease it prevents. Thus leaving a smaller proportion of unvaccinated individuals susceptible to the disease and possibly affecting transmission rates and reduce overall incidence of the disease within both groups. This would distort the study findings and not give a true picture of vaccine efficacy.
However if the unit of randomisation was the whole village and other villages were also recruited, villages would be randomised to the vaccine arm or standard care arm. This would allow for an analysis between arms which would more closely indicate the true efficacy of the vaccine.
Concept Check 1
Researchers were interested in investigating the intervention of sex education lessons given by medical students verses a control of standard sex education lessons in a population of early secondary school children and followed up for the rate of teenage pregnancy and STI’s.
Would a cluster randomised trial be an appropriate study design?
Please choose the correct statement/statements. The correct answer is at the bottom of the page.
A. Yes, because it would be difficult to randomise individuals receiving the intervention without them telling individuals in the control arm what they had learnt (contamination), thus making the groups more similar and preventing the efficacy of the intervention from being accurately determined.
B. Yes, because it would be logistically easier to send team of medical students to half of the schools to give sex education lessons than to all of them.
C. No, because a randomised controlled trial of individuals would be easier.
Considerations for trial design and analysis
A key consideration during the design of a cluster randomised trial is the similarity or correlation between individuals within a cluster, which could be a village, social group, region or administrative area.
For example if a poll was undertaken to estimate voting at the next UK general election a sample from the south of England might indicate a conservative win, in contrast a sample from Scotland might indicate a Scottish National Party win. That is individuals within communities may share similar cultural beliefs and indeed health related risk factors and outcomes, which makes the data obtained from a cluster correlated. This can be calculated as the intracluster correlation coefficient (ICC).
The practical implication is that the data obtained is not statistically independent, meaning that more participants overall are needed than for an individually randomised controlled trial. In addition the way the data is analysed is also different using a random effects model. The detail is not important for this tutorial, but the key consequence is that the effect estimate is less precise (wider confidence intervals).
Concept check 2
Researchers were interested in investigating the intervention of sex education lessons given by medical students verses a control of standard sex education lessons in a population of early secondary school children and followed up for the rate of teenage pregnancy and STI’s.
Data was analysed using a random effects model. Was this correct?
Please choose the correct statement/statements. The correct answer is at the bottom of the page.
A. No, because it’s a less precise estimate of the intervention effect.
B. Yes, because it makes allowance for the clustered data.
C. Yes, because all studies use random effects models.
Bringing it all together – Real world example
Neumark-Sztainer et al. (2000) investigated the effect of six 90 minute education sessions of media literacy and advocacy skills for the primary prevention of “disordered eating in pre-adolescent girls.
The study was undertaken in a population of 226 girls with a mean age of 10.6 from 24 scout groups. One of the outcomes was the Modified Sociocultural Attitudes Towards Appearance Questionnaire (SATAQ) at 3 months.
Question 1.
Was a cluster randomised trial an appropriate study design?
Please choose the correct statement/statements. The correct answer is at the bottom of the page.
A No, scout groups are not an appropriate unit of randomisation.
B No, there were 226 participants, which is enough for an individually randomised study.
C Yes, this study was ideally suited to a cluster randomised design due to groups already formed, which can be randomised to either the educational intervention or not.
Question 2.
The investigators described the statistical analysis in the abstract as “Performed t tests, chi squared tests, and analyses of covariance including troop as a random source of variation.”
Was this correct?
Please choose the correct statement/statements. The correct answer is at the bottom of the page.
A Yes, it was very thorough using several different tests.
B No, it did not take into account clustered data, which could be done using a random effects model.
C. No, non parametric tests should have been performed because of the small sample size.
Summary
Cluster randomised trials are a fascinating study design, which can be of particular use for educational and community level interventions. However there are many considerations and differences to the design, conduct and analysis in order to obtain valid results.
I do hope you enjoyed working through this and would appreciate any feedback on the content, design and presentational aspects of this tutorial.
References
Neumark-Sztainer D, Sherwood NE, Coller T, Hannan PJ. Primary prevention of disordered eating among preadolescent girls: feasibility and short-term effect of a community-based intervention. J Am Diet Assoc. 2000 Dec;100(12):1466-73.
Answers
Concept check 1. – Answers A and B are correct.
Concept check 2. – The correct answer is B.
Bringing it all together – Real world example. Question 1. – The correct answer is C.
Bringing it all together – Real world example. Question 2. – The correct answer is B.




No Comments on A 20 Minute Introduction to Cluster Randomised Trials
It is a brief summary of cluster randomisation and well organized with great examples. Thank you.
14th August 2018 at 8:06 amDear Hicks,
3rd February 2018 at 8:32 pmTo determine accuracy rate of clinicians for diagnosis of any disease in patiens, How I calculate the sample size. Can I decrease number of clinician needed with increasing patient number.
Thank you
Tim,
25th August 2013 at 10:16 pmYou need to distinguish whether random effects models are nested in the intervention condition (hierarchical models) or random effects “allowed for” in an observational study.
Thank you very much Peter for this post as well as our correspondence, I do hope that the following reasonably summarises this well enough and includes the two references suggested for greater detail of the statistical methods.
Tim
2 Key principles
“1) I think it important, and pedagogical, to distinguish between including random effects in a model for analysis of data from an observational study versus randomizing whole units in an experiment (trial, therefore a design issue), in which the units are NESTED in the experimental condition. Understanding that difference means understanding cluster randomized trials.”
“2) Why do a cluster randomized trial? I give only two reasons:
2nd September 2013 at 12:00 pmi) when the aim is to change group practice
ii) when the unit is the only way to deliver the intervention feasibly, without contamination.
I always mention “don’t do a cluster randomized trial if you don’t have to”, and I emphasize “do not do a cluster randomized trial for convenience or ease.”
Further reading:
Zucker D. An analysis of variance pitfall (the fixed effect analysis in a nested design). Educ Psych Meas. 1990;50:731–738
Murray E. In: Design and Analysis of Group-Randomized Trials. New York, NY: Oxford University Press; 1998;p. 9–15